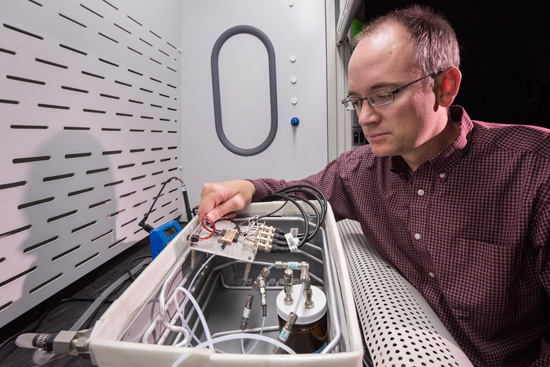
ALBUQUERQUE, N.M.: Scientists at the Sandia National Laboratories are working on explosive detection methods that would reliably detect homemade explosives, but will not be triggered by the ordinary materials like hydrogen peroxide used in these compounds. Hydrogen peroxide is found in everyday products ranging from soap, toothpaste and hair color to laundry bleach, carpet cleaners and stain removers. Defense-Update reports.
“Hydrogen peroxide explosives are a challenge because they are dangerous, but there are so many personal hygiene products that have hydrogen peroxide in them that the false positive rate is very high,” Chris Brotherton, principal investigator for a Sandia research project on chemiresponsive sensors explained. His work is built on field-structured chemiresistor technology developed at Sandia more than a decade ago by researchers James Martin and Doug Read. Chemiresistors are resistance-based sensors for volatile organic compounds. These materials allow users to tailor the sensors’ response range and sensitivity.
The research team at the lab is investigating methods to develop a portable sensor, able to detect a common homemade explosive called a FOx (fuel/oxidizer) mixture, made by mixing hydrogen peroxide with fuels. The detector must be able to spot hydrogen peroxide in concentrations that don’t also raise suspicions about common peroxide-containing products.
Brotherton’s Early Career Laboratory Directed Research and Development (LDRD) project proved a sensor could identify relatively high concentrations of hydrogen peroxide and differentiate that from a common interfering substance such as water, he said. The next step, Brotherton said, would be to work with an industrial partner to design an overall system that works faster and can be mass produced.
A major challenge was distinguishing between hydrogen peroxide and water, which exhibit similar behavior in chemiresistors. The key was choosing certain molecules in a polymer matrix, suggested by Brotherton’s Sandia technical mentor, polymer chemistry expert David Wheeler. When exposed to peroxide, those molecules react in a different way than when exposed to water.
The idea is to engineer the polymer to be as similar to the target material as possible, relying on the undergraduate rule that like dissolves like. For example, Wheeler said, if the target is a substance that’s not very polar, you’d choose a polymer with nonpolar groups. If the target has a lot of polarity, like water does, you’d develop polymers that could hydrogen-bond with water.
The tiny sensor incorporates the polymer and chains of miniscule conductive metal beads. The polymer reacts when it’s exposed to the substance being analyzed. “We tried to include specific molecules that would react with the peroxides,” Brotherton said.
Exposure to water also changes the polymer, but it returns to its previous state once the water is removed. Exposing the polymer to concentrated hydrogen peroxide, however, is irreversible. “So once you’ve done this to the polymer you’ve permanently changed it,” Brotherton said. “Instead of being a reusable sensor, it’s more of a disposable dosimeter.” According to Brotherto the proposed detector doesn’t react to toothpaste and other common peroxide products, thus making it more efficient for passenger inspection in airport security applications.
The sensor isn’t a silver bullet, but Brotherton said the technology has shown good results. “It has some challenges that have to be overcome, but we think it’s worth pursuing to the next level,” he said. For instance, the chemical reaction time must be reduced, so the sensor can be useful at a checkpoint. The detector also must be incorporated into a larger unit that includes equipment to gather a sample for analysis. “This technology would be easier to integrate into other detection technologies without impacting them too significantly,” he said. Such detectors, already available on the market today are about the size of a small, handheld vacuum cleaner. The support equipment would suck up a sample of air and the detector would test it.
“You’d need to know where the fumes were coming from,” Brotherton said. “It’s not enough to open up the whole room and suck in all the air and say, ‘There are peroxides somewhere in here, watch out.’ What we’d like to do is go up and down luggage, or be next to some sort of industrial process so we know this is most likely the source and it’s above a level we care about.”
Although a detector package could target a single type of vapor, a manufacturer could add it to a unit that detects several substances. That way, a checkpoint could have one sensing system rather than separate units for every material of concern, Brotherton suggested.
Source: Sandia National Laboratories/ Defense-Update.